The Effects of Periodized Traditional and Circuit-Based Resistance Training on Branched-Chain and Aromatic Amino Acid Metabolism and Ceramide Levels in Overweight and Obese Men
Keywords:
Amino acids, ceramide, insulin resistance, periodized resistance trainingAbstract
Objective: Amino acids (AAs) and their metabolites are altered with obesity and recognized as one of the predisposing factors for the development of insulin resistance. This study aimed to examine the impact of two types of resistance training on AAs, ceramide metabolism, and insulin resistance in overweight and obese men.
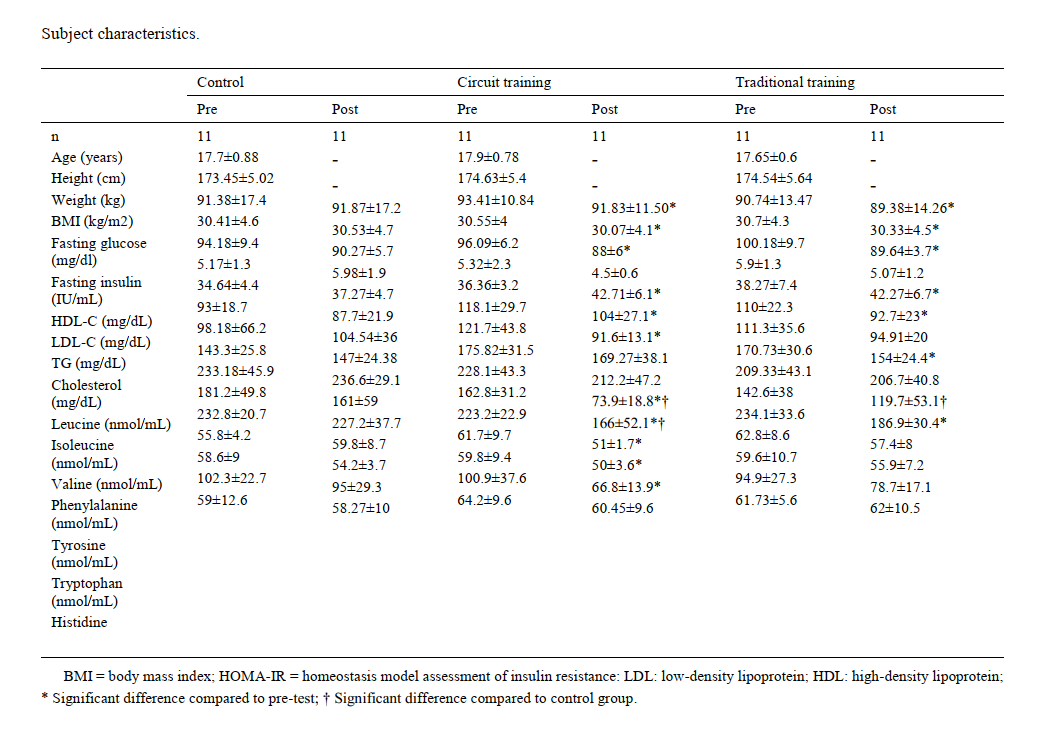
Methods and Materials: A total of 33 overweight and obese men were randomly divided into three control, circuit resistance training (CRT) and traditional resistance training (TRT) groups and the number of subjects in each group was 11 participants. The Training intervention consisted of periodized TRT and CRT training with the wave patterns and was conducted three sessions a week for three months (36 sessions). Serum levels of AAs and ceramides were measured using high-performance liquid chromatography (HPLC) at baseline and post-intervention.
Findings: The CRT group showed a notable reduction in total branched-chain amino acids (BCAAs), aromatic amino acids (AAAs), and ceramides compared to the control group (P = 0.001), indicating a meaningful metabolic improvement. Similarly, the TRT group exhibited moderate decreases in total BCAAs (P = 0.006) and AAAs (P = 0.017) relative to controls, changes that are inversely associated with insulin sensitivity. However, the differences between the CRT and TRT groups did not reach statistical significance, suggesting comparable effects between these two training protocols.
Conclusion: According to the results, the CRT group's changes were more significant than the TRT group. Therefore, circuit resistance training may prevent obesity-induced metabolic disorders.
Downloads
References
1. Tchkonia T, Thomou T, Zhu YI, Karagiannides I, Pothoulakis C, Jensen MD, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism. 2013;17(5):644-56.
2. Martin BC, Warram JH, Krolewski AS, Soeldner JS, Kahn CR, Bergman RN. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. The Lancet. 1992;340(8825):925-9.
3. Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Frontiers of Medicine. 2013;7(1):53-9.
4. Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends in pharmacological sciences. 2017;38(7):649-65.
5. Lee S, Gulseth HL, Langleite TM, Norheim F, Olsen T, Refsum H, et al. Branched-chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia. 2021;64(2):410-23.
6. Short KR, Chadwick JQ, Teague AM, Tullier MA, Wolbert L, Coleman C, et al. Effect of obesity and exercise training on plasma amino acids and amino metabolites in American Indian adolescents. The Journal of Clinical Endocrinology & Metabolism. 2019;104(8):3249-61.
7. Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched‐chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54(3):1063-70.
8. Evans WJ, Fisher EC, Hoerr RA, Young VR. Protein metabolism and endurance exercise. The Physician and Sportsmedicine. 1983;11(7):63-162.
9. Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Advances in nutrition. 2011;2(6):445-56.
10. Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends in Endocrinology & Metabolism. 2012;23(8):391-8.
11. Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45(1):42-72.
12. Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53(1):25-31.
13. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, DeFronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337-43.
14. Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. Journal of Applied Physiology. 2013;114(3):344-50.
15. Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, et al. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2013;98(4):1694-702.
16. Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. The Journal of Physiology. 2012;590(5):1049-57.
17. Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, et al. Muscle sphingolipids during rest and exercise: a C18: 0 signature for insulin resistance in humans. Diabetologia. 2016;59(4):785-98.
18. Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJF, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. American Journal of Physiology-Endocrinology and Metabolism. 2006;291(1):E99-E107.
19. Dobrzyń A, Górski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. American Journal of Physiology-Endocrinology And Metabolism. 2002;282(2):E277-E85.
20. Dube JJ, Amati F, Toledo FGS, Stefanovic-Racic M, Rossi A, Coen P, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147-56.
21. Helge JW, Dobrzyn A, Saltin B, Górski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Experimental Physiology. 2004;89(1):119-27.
22. Rivas DA, Morris EP, Haran PH, Pasha EP, Morais MdS, Dolnikowski GG, et al. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. Journal of applied physiology. 2012;113(11):1727-36.
23. Søgaard D, Baranowski M, Larsen S, Taulo Lund M, Munk Scheuer C, Vestergaard Abildskov C, et al. Muscle-saturated bioactive lipids are increased with aging and influenced by high-intensity interval training. International Journal of Molecular Sciences. 2019;20(5):1240.
24. Rasooli SA, Fathi R, Golzar FA-K, Baghersalimi M. The effect of circuit resistance training on plasma levels of amino acids, alpha-hydroxybutyrate, mannose, and urinary levels of glycine conjugated adducts in obese adolescent boys. Applied Physiology, Nutrition, and Metabolism. 2021;99(999):1-10.
25. Sayda MH, Phillips BE, Williams JP, Greenhaff PL, Wilkinson DJ, Smith K, et al. Associations between plasma branched chain amino acids and health biomarkers in response to resistance exercise training across age. Nutrients. 2020;12(10):3029.
26. Yano M, Kishida E, Muneyuki Y, Masuzawa Y. Quantitative analysis of ceramide molecular species by high performance liquid chromatography. Journal of Lipid Research. 1998;39(10):2091-8.
27. Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC medical research methodology. 2011;11(1):1-10.
28. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. American Journal of Physiology-Endocrinology and Metabolism. 2008;294(1):E15-E26.
29. Foschini D, Araújo RC, Bacurau RF, De Piano A, De Almeida SS, Carnier J, et al. Treatment of obese adolescents: the influence of periodization models and ACE genotype. Obesity. 2010;18(4):766-72.
30. Inoue DS, De Mello MT, Foschini D, Lira FS, Ganen ADP, Campos RMDS, et al. Linear and undulating periodized strength plus aerobic training promote similar benefits and lead to improvement of insulin resistance on obese adolescents. Journal of Diabetes and Its Complications. 2015;29(2):258-64.
31. Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. European Journal of Endocrinology. 2007;157(5):625-32.
32. McCann JR, Yang C, Bihlmeyer N, Tang R, Truong T, An J, et al. Branched chain amino acid metabolism and microbiome in adolescents with obesity during weight loss therapy. medRxiv. 2025:2025-02.
33. Lee S, Gulseth HL, Langleite TM, Norheim F, Olsen T, Refsum H, et al. Branched-chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia. 2021;64(2):410-23.
34. Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento-Villareal R, Sun Z, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metabolism. 2019;30(2):261-73.
35. Shou J, Chen PJ, Xiao WH. The effects of BCAAs on insulin resistance in athletes. Journal of Nutritional Science and Vitaminology. 2019;65(5):383-9.
36. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, DeFronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337-43.
37. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. The FASEB Journal. 2005;19(3):1-24.
Downloads
Additional Files
Published
Submitted
Revised
Accepted
Issue
Section
License
Copyright (c) 2025 Mehdi Changizi (Corresponding Author); Rozita Fathi, Rostam Alizadeh, Seyed Mohsen Avandi, Ali Khalghian (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.