The Effect of 8 Weeks of High-Intensity Interval Training on GLUT4 and GALR2 Gene Expression in Skeletal Muscle Tissue of Obese Female Rats with Type 2 Diabetes
Keywords:
high-intensity interval training, type 2 diabetes, GALR2, GLUT4Abstract
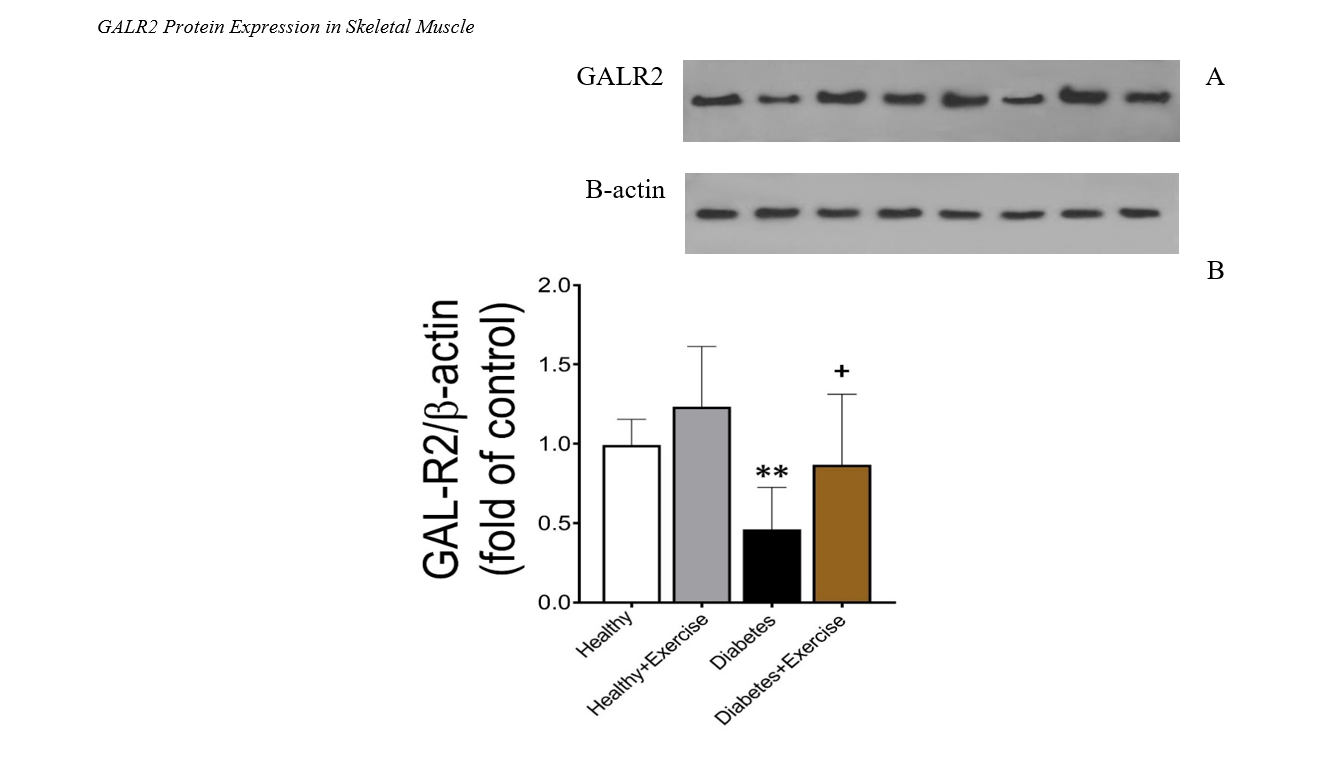
The present study is an applied, basic, and experimental research that examined the effect of 8 weeks of high-intensity interval training on the gene expression of GLUT4 and GALR2 in the skeletal muscle tissue of obese female rats with type 2 diabetes. To conduct this study, 40 female Wistar rats, aged 8 weeks with an average weight of 200 grams, were obtained from the animal farm of Kerman University of Medical Sciences for use in laboratory experiments at the same institution. After the animals adapted to their new environment, they were divided into four groups, with 10 rats each. The groups were as follows: healthy control (without diabetes and without exercise), diabetic control (with diabetes and without exercise), healthy exercise (without diabetes and with exercise), and diabetic exercise (with diabetes and with exercise). The diabetic control and diabetic exercise groups were induced with type 2 diabetes mellitus (T2DM) and then underwent an 8-week exercise intervention. To induce diabetes in the samples, a single intraperitoneal injection of 35 mg/kg streptozotocin (STZ) was administered. High-intensity interval training was conducted for 8 weeks, three sessions per week. Data analysis was performed using two-way ANOVA, and Tukey's post hoc test was used for intergroup comparisons. The findings showed that 8 weeks of high-intensity interval training led to a significant increase in the expression of GALR2 and GLUT4 in the gastrocnemius muscle tissue. Overall, it can be stated that 8 weeks of high-intensity interval training resulted in significant improvements in metabolism and hormonal function in obese female rats with type 2 diabetes. These exercises increased the expression of genes related to lipid and glucose metabolism, such as GALR2 and GLUT4, in skeletal muscle tissue, indicating enhanced energy consumption and improved metabolic performance in these animals.
Downloads
References
1. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). Journal of diabetes research. 2020;2020(1):3920196. [PMID: 32832560] [PMCID: PMC7424491] [DOI]
2. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. International journal of molecular sciences. 2020;21(17):6275. [PMID: 32872570] [PMCID: PMC7503727] [DOI]
3. Cai L, Xu Z, Luo H, He Q, Diao L, Gui X, et al. The association between 5-HT1A binding and temporal lobe epilepsy: A meta-analysis of molecular imaging studies. Epilepsy Behavior. 2023;145:109354. [PMID: 37473654] [DOI]
4. Cunningham AL, Stephens JW, Harris DA. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathogens. 2021;13:1-13. [PMID: 34362432] [PMCID: PMC8343927] [DOI]
5. Bijata M, Wirth A, Wlodarczyk J, Ponimaskin E. The interplay of serotonin 5-HT1A and 5-HT7 receptors in chronic stress. Journal of Cell Science. 2024;jcs-262219. [PMID: 39279505] [PMCID: PMC11491811] [DOI]
6. Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nature reviews Gastroenterology & hepatology. 2021;18(9):599-612. [PMID: 33972770] [DOI]
7. Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutrition & diabetes. 2020;10(1):38. [PMID: 33257645] [PMCID: PMC7705738] [DOI]
8. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. The Lancet. 2022;400(10365):1803-20. [PMID: 36332637] [DOI]
9. McGlynn RP, Cui M, Brems B, Holbrook O, Booth RG. Development of 2-Aminotetralin-Type Serotonin 5-HT1 Agonists: Molecular Determinants for Selective Binding and Signaling at 5-HT1A, 5-HT1B, 5-HT1D, and 5-HT1F Receptors. ACS Chemical Neuroscience. 2023;15(2):357-70. [PMID: 38150333] [PMCID: PMC10797628] [DOI]
10. Gabryelska A, Karuga FF, Szmyd B, Białasiewicz P. HIF-1α as a mediator of insulin resistance, T2DM, and its complications: potential links with obstructive sleep apnea. Frontiers in Physiology. 2020;11:1035. [PMID: 33013447] [PMCID: PMC7509176] [DOI]
11. Perreault L, Skyler JS, Rosenstock J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nature Reviews Endocrinology. 2021;17(6):364-77. [PMID: 33948015] [DOI]
12. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nature Reviews Endocrinology. 2021;17(9):534-48. [PMID: 34172940] [DOI]
13. Bickle JG, Li Y, Millette A, Dixon R, Wu S, Arias EC, et al. 5-HT1A Receptors on Dentate Gyrus Granule Cells Confer Stress Resilience. Biological Psychiatry. 2024;95(8):800-9. [PMID: 37863245] [DOI]
14. Ernawati U, Wihastuti TA, Utami YW. Effectiveness of diabetes self-management education (DSME) in type 2 diabetes mellitus (T2DM) patients: Systematic literature review. Journal of Public Health Research. 2021;10(2):jphr-2021. [PMID: 33855427] [PMCID: PMC8129774] [DOI]
15. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nature Reviews Endocrinology. 2020;16(7):349-62. [PMID: 32398822] [DOI]
16. Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25(8):1987. [PMID: 32340373] [PMCID: PMC7221535] [DOI]
17. Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovascular diabetology. 2022;21(1):83. [PMID: 35614469] [PMCID: PMC9134641] [DOI]
18. Rajpal A, Rahimi L, Ismail‐Beigi F. Factors leading to high morbidity and mortality of COVID‐19 in patients with type 2 diabetes. Journal of diabetes. 2020;12(12):895-908. [PMID: 32671936] [PMCID: PMC7405270] [DOI]
19. Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Current diabetes reports. 2021;21:1-13. [PMID: 33742318] [PMCID: PMC8805985] [DOI]
20. Turkel I, Ozerklig B, Yazgan B, Ozenc AE, Kubat GB, Simsek G, et al. Systemic and tissue-specific spexin response to acute treadmill exercise in rats. Peptides. 2024;180:171281. [PMID: 39111593] [DOI]
21. Jia D, Zhang H, Liu T, Wang R. Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites. 2024;14(3):135. [PMID: 38535295] [PMCID: PMC10972279] [DOI]
22. Konitz C, Schwensfeier L, Predel HG, Brinkmann C. The Influence of Acute and Chronic Exercise on Appetite and Appetite Regulation in Patients with Prediabetes or Type 2 Diabetes Mellitus—A Systematic Review. Nutrients. 2024;16(8):1126. [PMID: 38674817] [PMCID: PMC11054589] [DOI]
23. Chen X, He H, Xie K, Zhang L, Cao C. Effects of various exercise types on visceral adipose tissue in individuals with overweight and obesity: A systematic review and network meta‐analysis of 84 randomized controlled trials. Obesity Reviews. 2024;25(3):e13666. [PMID: 38031812] [DOI]
24. Wan Y, Su Z. The Impact of Resistance Exercise Training on Glycemic Control Among Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biological Research For Nursing. 2024;0(0). [PMID: 38623887] [DOI]
25. Gambaro SE, Zubiría MG, Giordano AP, Castro PF, Garraza C, Harnichar AE, et al. Role of Spexin in White Adipose Tissue Thermogenesis under Basal and Cold-Stimulated Conditions. International Journal of Molecular Sciences. 2024;25(3):1767. [PMID: 38339044] [PMCID: PMC10855774] [DOI]
26. Jurysta C, Nicaise C, Giroix MH, Cetik S, Malaisse WJ, Sener A. Comparison of GLUT1, GLUT2, GLUT4 and SGLT1 mRNA expression in the salivary glands and six other organs of control, streptozotocin-induced and goto-kakizaki diabetic rats. Cellular Physiology and Biochemistry. 2013;31(1):37-43. [PMID: 23343648] [DOI]
27. Chou SW, Chiu LL, Cho YM, Ho HY, Ivy JL, Ho CF. Effect of systemic hypoxia on GLUT4 protein expression in exercised rat heart. The Japanese journal of physiology. 2004;54(4):357-63. [PMID: 15631691] [DOI]
28. Neufer PD, Shinebarger MH. Effect of training and detraining on skeletal muscle glucose transporter (GLUT4) content in rats. Can J Physiol Pharmacol. 1992;70:1286-90. [PMID: 1493596] [DOI]
29. Lehnen A, Leguisamo N, Pinto G, Markoski M, De Angelis K. Exercise-stimulated GLUT4 Expression is Similar in Normotensive and Hypertensive Rats. Horm Metab Res. 2011;43:231-5. [PMID: 21332027] [DOI]
30. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. Jama. 2010;304(20):2253-62. [PMID: 21098771] [PMCID: PMC3174102] [DOI]
Downloads
Additional Files
Published
Submitted
Revised
Accepted
License
Copyright (c) 2024 Hanieh Faryabi (Author); Forouzan Fattahi Masrour (Corresponding Author); Maghsoud Peeri (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.